Describe the Structure of Sodium Chloride
A common sulfide mineral that crystallizes in this manner is the ore mineral of lead galena. Must include type and magnitude of charge for each ion 1 electrostatic attraction allow attraction between opposite charges 1 b.
Sodium Chloride Structure Properties And Uses Of Nacl
It is widely known as table salt and is mostly used in the food industry for preservation and flavouring.

. Sodium chloride has a molar mass of 5844 grams per mole. Its highly symmetric form consists of cubes modified by octahedral faces at their corners. It appears as a white crystalline solid and is usually odorless in nature.
From the difference in intensity of reflections 0f alternate orders it was concluded that sodium chloride lattice consists of interpenetrating face-centred cubic lattice of sodium and chloride ions. Crystal Structure of Sodium Chloride Na Cl. What are the products of the reaction between chlorine and water.
Ionic crystals depend upon the structure and the size of their ions. Then draw an identical square offset from the first one. How to draw a simple sodium chloride lattice.
Chemistry questions and answers. The structure of sodium chloride. The salient features of its structure are.
Sodium chloride composed of two ions Na and Cl- is an ionic compound having the chemical formula NaCl. It is also available as aqueous solutions with different concentrations which are known as saline solutions. Sodium chloride is an ionic compound.
Sodium chloride ˌsoʊdiəm ˈklɔːraɪd commonly known as salt is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. The crystal lattice of sodium chloride is shown in Figure 46. The following figure shows how these ions are arranged in the crystal lattice.
NaCl is the molecular formula of sodium chloride and 5844 g mol is its molar mass. It included 4 Na ions and 4 Cl ions. As a salt sodium chloride dissolves well in water and the ions in the crystals will separate when in solution.
This is the diagram we are aiming at. The classic case of ionic bonding the sodium chloride molecule forms by the ionization of sodium and chlorine atoms and the attraction of the resulting ions. Crystalline Structure Of Sodium Chloride Definition.
Sodium chloride a white crystalline solid contains a density of 2165 gmL a melting point of 801 C and a boiling point is about 1413 C. Sodium chlorine would form an ionic bond which is the transfer of electrons because sodium is in group 1 it has 1 electron on its outermost shell because chlorine is in group 7 it has 7 electrons on the outermost shell. This page is an add-on to a page about giant ionic structures.
Sodium ions are so located that there are six chloride ions around it. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of. These ionic bonds form when the less electronegative sodium.
Each sodium ion is surrounded by 6 chloride ions. In the structure of NaCl each Na ion is surrounded by six chloride ions. Microscopic View Describe the atomic microscopic perspective of a sodium chloride crystal as shown in the computer modeling software.
Which statements best describe the structure of sodium chloride NaCl. Sodium chloride 2pts Describe the microscopic perspective of sodium chloride. Register with BYJUS to learn more on the topic and several other topics of chemistry.
This compound is water-soluble and consists of sodium cation and chloride anion. It simply describes how to draw the structure of a sodium chloride lattice. In the lattice each sodium ion is surrounded by six chloride ions and vice versa co-ordination number is six.
This equivalent to saying that sodium ions occupy. The bonding in sodium chloride. There are 4 octahedral voids.
The chloride ions are arranged octahedrally around each sodium ion. Architecture of the sodium chloride structure. Structure of Sodium Chloride Chemistry Skills.
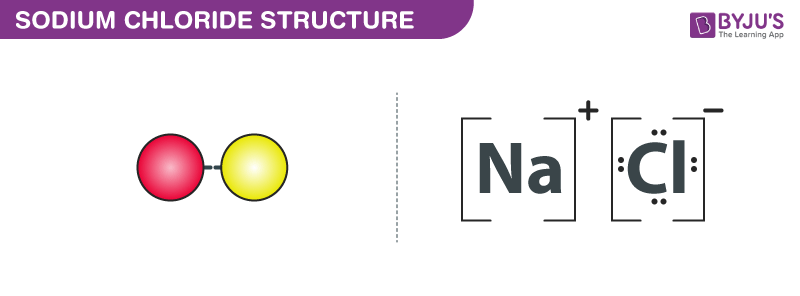
The chemical formula for sodium chloride is NaCl which means that for every sodium atom present there is exactly one chloride atom. In this article we will discuss sodium chloride NaCl lewis dot structure. Ionic compounds such as sodium chloride are arranged in a giant three-dimensional lattice.
With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. First draw a perfect cube. I ii and iii.
The chemical formula for sodium chloride is NaCl which means that for every sodium atom present there is exactly one chloride atom. It is an ionic compound which consists of a chloride anion Cl- and a sodium cation Na. 1 Page 8 2 Bonding Big Sheet Answers M1a lattice giant structure max 3 if incorrect structure or bonding or particles 1 ionic or contains ions 1 Na and Claccept in words or dot and cross diagram.
It is made of sodium ions which have lost an electron to become positively charged Na and chloride ions which have gained an electron to become negatively charged Cl-. The molecular weight of NaCl is 5844gmol. An atom of sodium has one 3s electron outside a closed shell and it takes only 514 electron volts of energy to remove that electron.
The ions are held together in the lattice by strong ionic bonds between the oppositely charged sodium and chloride ions. 9 rows Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride. The sodium and chloride ion are present in the ratio 11.
It appears as a solid clear crystal with little or no odor. The lattice form a cubic structure. Sodium chloride is a giant ionic lattice consisting of alternating adjacent positively charged sodium ions and negatively charged chloride ions in a regular arrangement of rows and columns.
The most common use of sodium chloride is in the Solvay process for the production of sodium carbonate and calcium chloride. The crystalline structure of Sodium Chloride is a face-centered cubic. State and describe the structure bonding and properties in Sodium Chloride.
Sodium chloride is made up of sodium and chlorine. The pH of sodium chloride is 7. The total number of ions present in one unit cell of sodium chloride is 8.
Chloride ions are ccp type of arrangement ie it contains chloride ions at the corners and at the center of each face. Sodium chloride has a molar mass of 5844 grams per mole.

Structure Of Nacl Sodium Chloride Youtube

Sodium Chloride Nacl Crystal Physicsopenlab
What Is The Type Of Lattice Structure Of Nacl Quora
What Is The Structure Of Sodium Chloride In A Solid State Quora
No comments for "Describe the Structure of Sodium Chloride"
Post a Comment